Supplier of Oil Products and Petrochemical
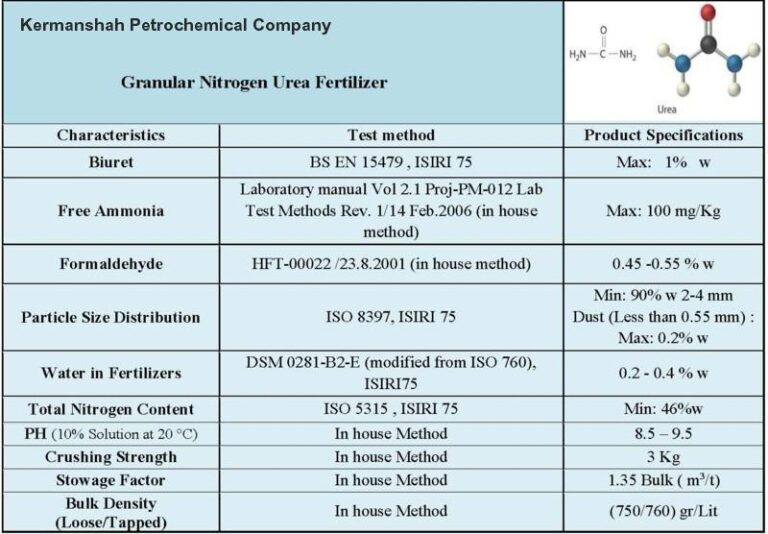
Urea 46
“Urea 46” likely refers to urea fertilizer with a nitrogen content of 46%.
In agricultural contexts, urea fertilizer is often sold based on its nitrogen content, with “46” indicating that the fertilizer contains 46% nitrogen by weight. This makes it one of the highest nitrogen-content solid fertilizers available.
Urea fertilizer is widely used in agriculture due to its high nitrogen content and relatively low cost compared to other nitrogen fertilizers. It provides an efficient source of nitrogen for plant growth, as nitrogen is an essential nutrient required for the synthesis of proteins and other vital molecules in plants.
When applied to soil, urea undergoes hydrolysis by soil enzymes to release ammonium (NH4+) and bicarbonate ions (HCO3-), which can then be converted to plant-available forms of nitrogen, such as ammonium (NH4+) and nitrate (NO3-), through microbial processes.
Overall, urea 46 is a common and widely used nitrogen fertilizer in agricultural production systems.
Analyzing “Urea 46” typically involves understanding its composition, properties, and applications. Here’s an analysis of Urea 46:
- Composition: Urea 46 refers to urea fertilizer with a nitrogen content of 46%. Urea is a chemical compound with the formula CO(NH2)2. It is a white, crystalline solid that contains 46% nitrogen by weight, making it one of the highest nitrogen-content solid fertilizers available.
- Properties: Urea 46 is highly soluble in water, which allows for easy application to soil or crops. It provides a concentrated source of nitrogen, which is an essential nutrient for plant growth and development. Urea fertilizer is neutral in pH, making it suitable for a wide range of soil types and crops.
- Application: Urea 46 is commonly used in agriculture as a nitrogen fertilizer to promote plant growth, increase crop yields, and improve crop quality. It is suitable for use on a wide range of crops, including cereals, oilseeds, fruits, vegetables, and legumes. Urea can be applied to the soil surface or incorporated into the soil during planting.
- Nitrogen Release: Urea fertilizer undergoes hydrolysis in the soil, where it is converted into ammonium (NH4+) and bicarbonate ions (HCO3-). This process is facilitated by soil enzymes called ureases. The ammonium ions are subsequently converted into plant-available forms of nitrogen, such as ammonium (NH4+) and nitrate (NO3-), through microbial processes.
- Efficiency: Urea 46 is known for its high nitrogen content, which provides an efficient and cost-effective source of nitrogen for plants. However, its efficiency depends on factors such as soil conditions, climate, crop type, and application methods. Proper management practices, including accurate application rates and timing, are essential to maximize the effectiveness of urea fertilizer.
- Environmental Considerations: While urea fertilizer is widely used in agriculture, its application can lead to environmental concerns such as nitrogen leaching, runoff, and nitrous oxide emissions. Proper application practices, including split applications and the use of nitrogen stabilizers, can help minimize these environmental impacts.
In summary, Urea 46 is a high-nitrogen fertilizer commonly used in agriculture to promote plant growth and increase crop yields. Its properties, application methods, and environmental considerations make it a valuable tool for farmers worldwide. However, proper management practices are essential to ensure its effective and sustainable use in crop production systems.
“Urea 46” likely refers to urea fertilizer with a nitrogen content of 46%.
In agricultural contexts, urea fertilizer is often sold based on its nitrogen content, with “46” indicating that the fertilizer contains 46% nitrogen by weight. This makes it one of the highest nitrogen-content solid fertilizers available.
Urea fertilizer is widely used in agriculture due to its high nitrogen content and relatively low cost compared to other nitrogen fertilizers. It provides an efficient source of nitrogen for plant growth, as nitrogen is an essential nutrient required for the synthesis of proteins and other vital molecules in plants.
When applied to soil, urea undergoes hydrolysis by soil enzymes to release ammonium (NH4+) and bicarbonate ions (HCO3-), which can then be converted to plant-available forms of nitrogen, such as ammonium (NH4+) and nitrate (NO3-), through microbial processes.
Overall, urea 46 is a common and widely used nitrogen fertilizer in agricultural production systems.
Analyzing “Urea 46” typically involves understanding its composition, properties, and applications. Here’s an analysis of Urea 46:
- Composition: Urea 46 refers to urea fertilizer with a nitrogen content of 46%. Urea is a chemical compound with the formula CO(NH2)2. It is a white, crystalline solid that contains 46% nitrogen by weight, making it one of the highest nitrogen-content solid fertilizers available.
- Properties: Urea 46 is highly soluble in water, which allows for easy application to soil or crops. It provides a concentrated source of nitrogen, which is an essential nutrient for plant growth and development. Urea fertilizer is neutral in pH, making it suitable for a wide range of soil types and crops.
- Application: Urea 46 is commonly used in agriculture as a nitrogen fertilizer to promote plant growth, increase crop yields, and improve crop quality. It is suitable for use on a wide range of crops, including cereals, oilseeds, fruits, vegetables, and legumes. Urea can be applied to the soil surface or incorporated into the soil during planting.
- Nitrogen Release: Urea fertilizer undergoes hydrolysis in the soil, where it is converted into ammonium (NH4+) and bicarbonate ions (HCO3-). This process is facilitated by soil enzymes called ureases. The ammonium ions are subsequently converted into plant-available forms of nitrogen, such as ammonium (NH4+) and nitrate (NO3-), through microbial processes.
- Efficiency: Urea 46 is known for its high nitrogen content, which provides an efficient and cost-effective source of nitrogen for plants. However, its efficiency depends on factors such as soil conditions, climate, crop type, and application methods. Proper management practices, including accurate application rates and timing, are essential to maximize the effectiveness of urea fertilizer.
- Environmental Considerations: While urea fertilizer is widely used in agriculture, its application can lead to environmental concerns such as nitrogen leaching, runoff, and nitrous oxide emissions. Proper application practices, including split applications and the use of nitrogen stabilizers, can help minimize these environmental impacts.
In summary, Urea 46 is a high-nitrogen fertilizer commonly used in agriculture to promote plant growth and increase crop yields. Its properties, application methods, and environmental considerations make it a valuable tool for farmers worldwide. However, proper management practices are essential to ensure its effective and sustainable use in crop production systems.